The damaging effects of excessive complement activation

Excessive activation of the complement system may accelerate cell damage in the area just outside the lesion,
increasing the risk of Geographic Atrophy (GA) lesion growth1-4
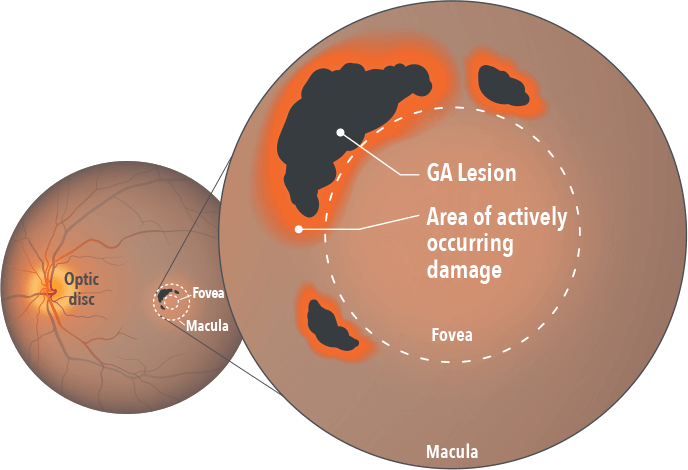
The complement system normally plays a pivotal role in the immune system’s defense against pathogens and abnormal cells.5 However, in patients with GA, increased levels of complement activity have been found not just in the lesion, but in the area just outside of it.4,6
This excessive activation of the complement system can lead to excess phagocytosis, inflammation, and cell lysis – all of which culminate in retinal cell death.1,5
See the consequences of excessive complement activation in GA
This is Geographic Atrophy, or GA, when damage to the retina is irreversible. To uncover what’s behind the destruction, let’s look below the surface.
In this advanced form of dry age-related macular degeneration, atrophic lesions form on the macula, causing loss of functional vision as they spread. This group of proteins is called the complement system. Normally, they defend against pathogens and remove abnormal cells, yet in Geographic Atrophy, or GA, too much complement activity can be detrimental.
This central protein, C3, plays a key role in the excessive complement activity. Complement activation causes C3 to cleave, splitting it apart into the active fragments, C3a and C3b. Over by this photoreceptor, a group of C3a’s have begun to accumulate. Too much C3a can trigger chronic inflammation of the macula that can contribute to cell death.
Here, a group of C3b’s are accumulating on cell surfaces, tagging them for phagocytosis by immune cells. But the journey is far from over for C3b, which also has significant effects on the complement cascade, causing downstream components to work together and form something new, the membrane attack complex, that latches onto and forms an opening in the cellular membranes of retinal pigment epithelial cells, or RPE, as well as photoreceptors.
C3 is a central protein in the complement system, but when there is excessive complement activation, C3 plays a key role in inflammation, phagocytosis, and cell membrane disruption.
All of which are thought to ultimately lead to retinal cell death.
Excessive activation of the complement system has been associated with lesion growth in GA4
C3 plays a central role in driving the multiple damaging downstream effects of excessive complement activity in GA that can lead to retinal cell death4,7-9
C3 is the linchpin of excessive complement activation in GA4,7,8
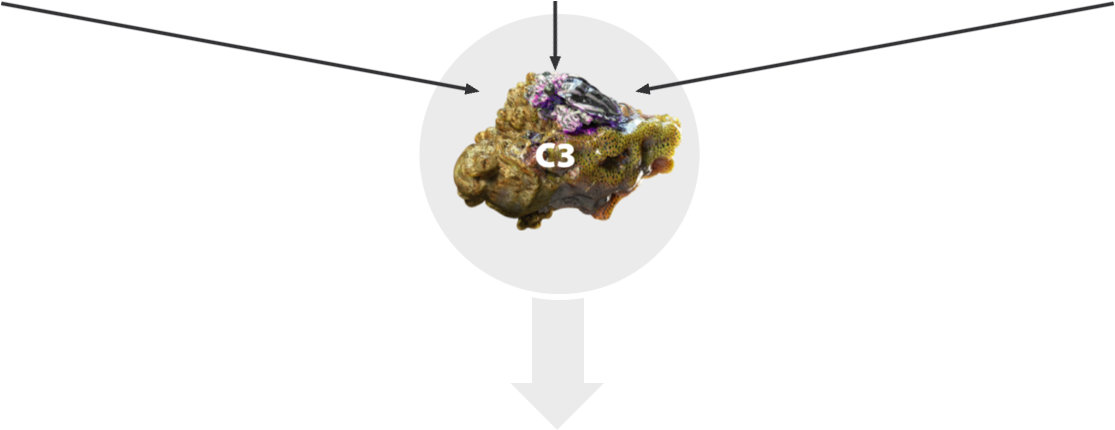
C3b is involved in an amplification loop for complement activation4,10
All three complement pathways converge at C3, leading to cleavage of C3 into components C3a and C3b and downstream effects3

Activation and recruitment of inflammatory cells (by C3a and downstream protein C5a)5,6,9
Complement accumulation labeling cells for phagocytosis (by C3b)5,6,10
Cell membrane disruption from membrane attack complex formation (by downstream proteins C5b-9)7,8

Hear from
your peers
Experts share their insights and experiences managing patients with GA.

Sign up to stay informed!
Connect with us to receive updates and information from Apellis.

References:
- Boyer DS, Schmidt-Erfurth U, van Lookeren Campagne M, et al. The pathophysiology of geographic atrophy secondary to age-related macular degeneration and the complement pathway as a therapeutic target. Retina. 2017;37(5):819-835. doi:10.1097/iae.0000000000001392.
- Park DH, Connor KM, Lambris JD. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10:1007. doi:10.3389/fimmu.2019.01007.
- Yates JRW, Sepp T, Matharu BK, et al. Genetic Factors in AMD Study Group. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553-561. doi:10.1056/NEJMoa072618.
- Katschke KJ Jr, Xi H, Cox C, et al. Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep. 2018;8(1):7348. doi:10.1038/s41598-018-25557-8.
- Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I – Molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. doi:10.3389/fimmu.2015.00262.
- Smailhodzic D, Klaver CC, Klevering BJ, et al. Risk alleles in CFH and ARMS2 are independently associated with systemic complement activation in age-related macular degeneration. Ophthalmology. 2012;119(2):339-346. doi:10.1016/j.ophtha.2011.07.056.
- Mastellos DC, Reis ES, Ricklin D, Smith RJ, Lambris JD. Complement C3-targeted therapy: replacing long-held assertions with evidence-based discovery. Trends Immunol. 2017;38(6):383-394. doi:10.1016/j.it.2017.03.003.
- Ricklin D, Reis ES, Mastellos DC, Gros P, Lambris JD. Complement component C3 – The “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274(1):33-58. doi:10.1111/imr.12500.
- Heesterbeek TJ, Lechanteur YTE, Lorés-Motta L, et al. Complement activation levels are related to disease stage in AMD. Invest Ophthalmol Vis Sci. 2020;61(3):18. doi:10.1167/iovs.61.3.18.
- Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45(11):1366-1370. doi:10.1038/ng.2741.
